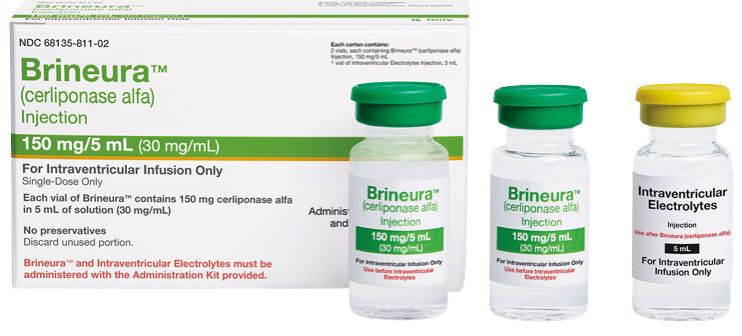
Description
Brineura (cerliponase alfa) is an enzyme replacement therapy used to treat neuronal ceroid lipofuscinosis type 2 (CLN2), also known as late infantile Batten disease. It is a rare, inherited neurodegenerative disorder caused by a deficiency of the enzyme tripeptidyl peptidase 1 (TPP1), leading to the accumulation of waste materials in the brain’s cells.
Brineura works by delivering a synthetic form of the missing TPP1 enzyme directly into the cerebrospinal fluid through an intraventricular infusion, helping to slow the loss of motor function in affected children. It was developed by BioMarin Pharmaceutical and approved by the U.S. FDA in 2017 as the first treatment for CLN2 disease.


Reviews
There are no reviews yet.